IntroductionCMVinfectioninahealthyhumanisusuallysubclinical.However,intheimmuno-compromisedhostandadevelopingfoetusitmayresultineitheralocalizedordisseminateddisease.ClinicalmanifestationsofCMVdiseaseincludepneumonia,retinitis,hepatitis,enteritisandneurologicaldisease.Despiteimprovedtreatmentmodalities,CMVinfectionmayresultinsignificantmorbidityandmortality.PatientsareatriskfrombothprimaryCMVinfectionandreactivationoflatentinfection.
TheCMVpp65antigenemiaassayisavaluabletoolinthediagnosisandmonitoringofactiveCMVinfectioninsolidorganandbonemarrowtransplantpatientsaswellasinthediagnosisandmonitoringofCMVdiseaseinAIDSpatients.EarlyandrapiddiagnosisofactiveCMVinfectionisofgreatimportanceinavoidingover-treatmentwithimmunosuppressivedrugsandinguidingantiviraltherapy.
ThestandardinCMVtesting,antigenemia,isanon-culturetechniquethatdetectsanactiveinfectionwithbloodsampleanalysisandisoptimizedforuseintheCMVBrite™KitandCMVBrite™TurboKit.
TheCMVBrite™TurboantigenemiakitusesthewelldefinedC10/C11antibodycocktailtodetecttheCMVlowermatrixphosphoprotein(pp65),anearlyantigeninvirusreplication,whichisabundantlypresentinantigen-positivepolymorphonuclearcells.TheCMVBrite™TurboKit,isarapidnewversionofthefirstFDAregisteredimmunofluorescenceantigenemiakitforinvitroCMVdiagnosis.
PrincipleoftheCMVBrite™TurboKitTheCMVantigenemiaassayhasbeendevelopedusingacocktailoftwomonoclonalantibodies(C10/C11)directedagainstCMVlowermatrixproteinpp65.TheassayusestheC10/C11cocktailinanindirectimmunofluorescencestainingofcytospinpreparationsofperipheralbloodleukocytes.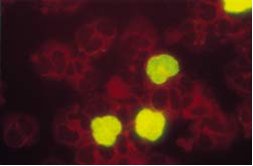 Figure:HumanperipheralbloodleukocytesfromapatientwithanactiveCMVinfection,stainedwiththeCMVBriteantigenemiakit.ImmunofluorescencestainingofCMVpp65antigenpositivepolymorphnuclearcells.Negativecellsarecounterstained(red)withEvansBlue.
Figure:HumanperipheralbloodleukocytesfromapatientwithanactiveCMVinfection,stainedwiththeCMVBriteantigenemiakit.ImmunofluorescencestainingofCMVpp65antigenpositivepolymorphnuclearcells.Negativecellsarecounterstained(red)withEvansBlue.














